Problem Statement
In today’s global pharmaceutical supply chain, managing product pedigrees across multi-level production by time periods (years, quarters, months) is critical for ensuring compliance, optimizing resources, and meeting market-specific demands.
Pharmaceutical production often involves multiple steps spanning primary sites for API (Active Pharmaceutical Ingredient) manufacturing and secondary sites for filling and packaging. Ensuring compliance with market-specific regulations and managing the use of specific production pedigrees—such as CMOs (Contract Manufacturing Organizations), production lines, and intermediate materials—can be complex. Misalignment in pedigree management may lead to inefficiencies, regulatory non-compliance, and wasted resources, hampering the ability to meet global market demand.
Solution Statement
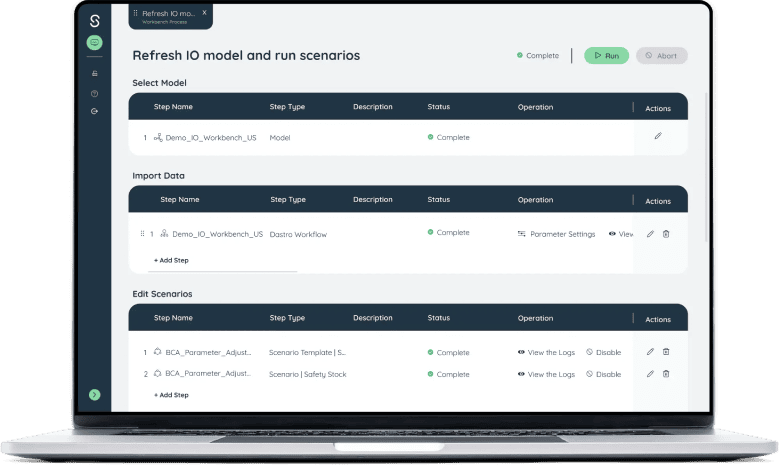
Sophus provides a dynamic, end-to-end solution for optimizing the pharmaceutical supply chain. It enables companies to manage and restrict the use of specific pedigrees based on market demand and time periods, ensuring compliance with regulatory and market-specific requirements. By aligning production across primary and secondary sites, Sophus helps pharmaceutical companies maintain traceability, optimize resource allocation, and deliver precision in meeting global demand across complex, multi-level production processes.